We study how single-cell embryos localize RNAs and proteins to pattern developmental potential. We use genetics, microscopy and biochemistry to identify, observe and manipulate RNAs and proteins in and out of cells.
Cell Polarity
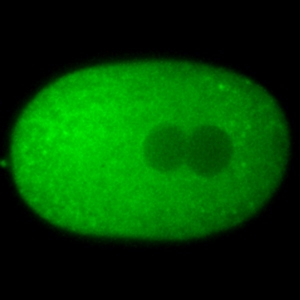
Polarization of the cytoplasm and asymmetric cell division are common strategies to pattern cells and embryos. We study the mechanisms that polarize the C. elegans embryo right after fertilization. We have found that localized changes in the rates of protein diffusion drive the formation of protein gradients across the cytoplasm. We want to understand the mechanisms that limit and patter protein diffusion in the cytoplasm.
RNA Granules
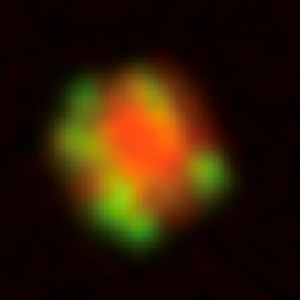
RNA granules are protein/RNA condensates proposed to form by liquid-liquid phase separation, a process akin to the separation of oil and vinegar in salad dressing. Studying the P granules of C. elegans, we have found that RNA granules are stabilized in the cytoplasm by a gel-like coat. The coat is made by intrinsically-disordered proteins that are stimulated by RNA to form gels in vitro. Similar proteins exist across eukarya, raising the possibility that protein gels may be a common strategy to stabilize liquid condensates in eukaryotic cells.
Germ Cells
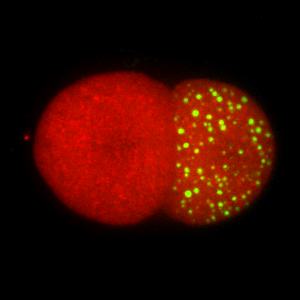
One of the first lineage to arise during embryogenesis is the germline, the lineage that gives rise to oocytes and sperm. The embryonic germline exhibits several unique features, including delayed activation of transcription in the nucleus and large RNA granules in the cytoplasm. We are studying how these features make the germline unique and different from all other cell types in our bodies.
CRISPR Genome Editing
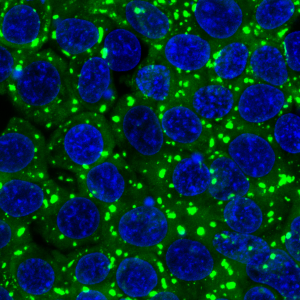
A key tool for any biologist today is the ability to edit the genome. CRISPR-Cas9 is an RNA-guided endonuclease that can be programmed to cut any gene in the genome. We have developed a simple protocol to repair Cas9-induced cuts using linear templates created by PCR. Our method can be used to edit the genome of human cells, mice and C. elegans. Genome editing is a technique that we use routinely in the lab, in particular to attach fluorescent tags to proteins so we can watch their dynamics live.